IPS cells passage on 6cm dish
- Aspirate medium
- Wash with PBS
- Put RT TRYPLE 1mL, incubate 2-4min at 37C until cells appear isolated (but not floating)
- Carefully aspirate TRYPLE, add 3mL of E8 and gently dissociate cells using 1000uL pipette tip, take 10uL of suspension for countess measurment (confluent 6cm dish has 9-11million cells)
- Spin 300g/3min
- Prepare E8 complete with 1:2000 RI (4mL for 6cm dish, 2mL 6well, 1mL 12 well, 0.5mL 24well)
- Discard supernatant and resuspend to concentration 2million cells/mL in E8+RI
- Put E8+RI in a new matrigel coated (1:33 MG + 1:100 Geltrex) dish, add cell suspension (200uL for 6cm dish, 100uL for 6well, 50uL for 12well, 25uL for 24well) to seed 20 000cells/cm2
- Rock to distribute cells evenly
- Change media daily (for first 3 days, change half of medium, spin the dish to congregate debris in the center of the dish to be aspirated)
- Passage when confluent (without borders between cells visible in monolayer, only cell nucleui should be visible)
Cardiac differentiation from monolayer
Note: The number of days before cells reach monolayer are variable, wait till you get full confluency with a good morphology
Day -4
- Wash confluent monolayer cells (the borders between cells are not visible, only the nuclei are visible) with PBS
- Put 1mL of TRYPLE in a dish, incubate for 2min (for PS)-to 5+ min, check visually for the moment when the cells get individualed but do not float yet
- gently aspirate tryple, add 3mL of E8, gently dissociate cells form surface, také aliquot for counting and transfer the rest in 15mL tube
- spin at 150g for 3 min, count cells the conc should be between 2.5-4 millions per mL =7.5-12millions of cells total
- discard supernatant, resuspend pellet in E8+RI (1to2000) to 2mil/mL concentration
- seed at density 30 000, 60 000 cells, 120000cells/cm2 in desired dish. I tested different concetrations, but these work best to get contractile monolayer, when cells are kept in the same dish. For 12w use 2mL of medium in a well.
Day -3,D-2, D-1
- change E8 medium daily, 2mL per well, be gentle when changing medium, the overconflluent monolayer can detach (mostly in higher concentrations than optimal)
Day 0
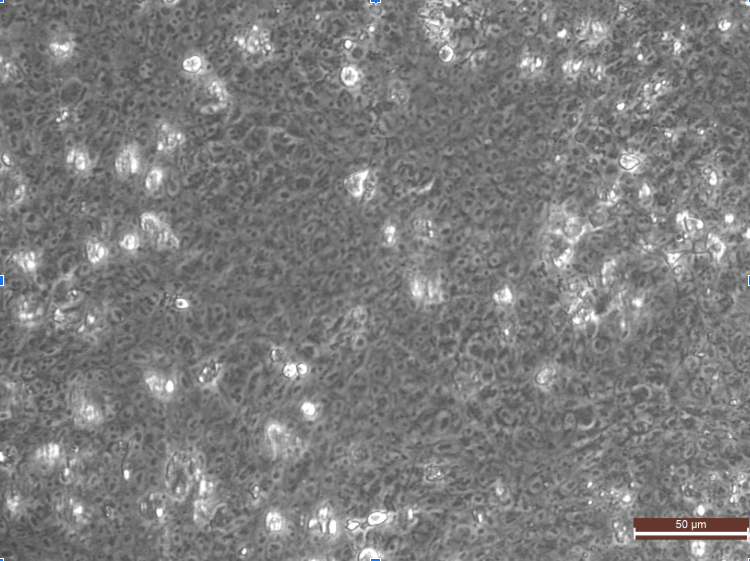
- in the morning check cells, cells should get totally confluent no holes in monolayer (see picture D0), no visible borders between cells, if this is not true, do not proceed but start over with higher concentration/better cells or wait longer*.
- change medium (2mL for 12well) to :
- RPMI
- B27w/out Ins+L (1to50)
- PenStrep (1to100)
- 8uM CHIR (1to1000)
- Geltrex (1to100), record time of media change
- In case of observed delamination of differentiating cells in early days of differentiation (YAP1-KO do it sometime) use 1to2000 Rock Inhibitor
Day 1 (optional, better for YAP1-KO)
- change medium (2mL for 12well) to :
- RPMI + L-Glut
- B27w/out Ins (1to50)
- PenStrep (1to100)
- Geltrex (1to100), record time of media change
Day 2
- Try to keep the time of change (morning/lunchtime/evening)
- change medium (2mL for 12well) to :
- RPMI + L-Glut
- B27w/out Ins (1to50)
- PenStrep (1to100)
- 5uM IWP2 (1to1000)
- Geltrex (1to100), record time of media change
Day 4
- Try to keep the time of change (morning/lunchtime/evening)
- change medium (2mL for 12well) to :
- RPMI + L-Glut
- B27w/out Ins (1to50)
- PenStrep (1to100)
- Geltrex (1to100), record time of media change
Day 6 (split can be performed)
- Try to keep the time of change (morning/lunchtime/evening)
- change medium (2mL for 12well) to :
- RPMI + L-Glut
- B27w/out Ins+ (1to50)
- PenStrep (1to100)
- Geltrex (1to100), record time of media change
- Rock Inhibitor (1to2000)
Day 8 onwards
Initiation of beating (D8-12 cells should start beating)
- Try to keep the time of change (morning/lunchtime/evening)
- up to day 16 : Check if the cells are beating if so, switch to medium with B27 containing Insulin
- If the cells do not beat at D16 => trash
Media change (every 2-3 days)
- change medium (2mL for 12well) to :
- RPMI + L-Glut
- B27 + (1to50)
- PenStrep (1to100)
Picture D0 of 0.031 seeding density